NCERT Solutions for Class 11 Physics Chapter 12 Thermodynamics will give you the self-confidence to face Class 11 examination and entrance examination.
This NCERT Solutions consists of answers to the questions provided in the textbook, important questions in previous year question papers and CBSE sample papers. Exercises like worksheets and exemplary problems are provided here to help the students to get tuned in with the topic Thermodynamics.
Download NCERT Solutions Class 11 Physics Chapter 12 PDF:-Download Here
Thermodynamics is the hot topic for the experts who prepare question papers. In order to get more marks in Class 11 Physics, it is crucial to study these solutions as one can expect many questions from this resource being asked often in entrance exams and competitive exams.
Thermodynamics is one of the most scoring sections in Class 11 Physics. Students must study this Chapter in-depth to excel in the exam. Some key points of Thermodynamics are given below.
Equilibrium in thermodynamics refers to the situation when macroscopic variables describing the thermodynamic state of a system does not depend on time. Equilibrium of a system in mechanics means the net external force and torque on the system are zero. The temperature of a body is related to its average internal energy, not to the kinetic energy of motion of its centre of mass. A bullet fired from a gun is not at a higher temperature because of its high speed.
Heat capacity, in general, depends on the process of the system that goes through when the heat is supplied.
In a state of thermodynamic equilibrium, the microscopic constituents of a system are not in equilibrium
In isothermal quasi-static processes, heat is absorbed or given out by the system even though at every stage the gas has the same temperature as that of the surrounding reservoir. This is possible because of the infinitesimal difference in temperature between the system and the reservoir.
NCERT Solutions for Class 11 Physics Chapter 12 Thermodynamics
Subtopics in NCERT Solutions for Class 11 Physics Chapter 12 Thermodynamics:
| Section Name | Topic Name |
| 12 | Thermodynamics |
| 12.1 | Introduction |
| 12.2 | Thermal equilibrium |
| 12.3 | Zeroth law of thermodynamics |
| 12.4 | Heat, internal energy and work |
| 12.5 | First law of thermodynamics |
| 12.6 | Specific heat capacity |
| 12.7 | Thermodynamic state variables and equation of state |
| 12.8 | Thermodynamic processes |
| 12.9 | Heat engines |
| 12.10 | Refrigerators and heat pumps |
| 12.11 | Second law of thermodynamics |
| 12.12 | Reversible and irreversible processes |
| 12.13 | Carnot engine |
QUESTIONS FROM TEXTBOOK
Question 12. 1 A geyser heats water flowing at the rate of 3.0 litres per minute from 2 7°C to 77°C. If the geyser operates on a gas burner, what is the rate of consumption of the fuel if its heat of combustion is 4.0 x 104 J/g?
Answer: Volume of water heated = 3.0 litre per minute Mass of water heated, m = 3000 g per minute Increase in temperature,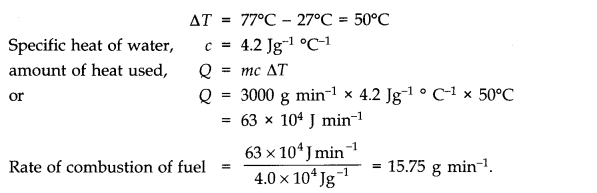
Question 12. 2 What amount of heat must be supplied to 2.0 x 10-2 kg of nitrogen (at room temperature) to raise its temperature by 45 °C at constant pressure? (Molecular mass of N2 = 28; R = 8.3 J mol-1 K-1.)
Answer: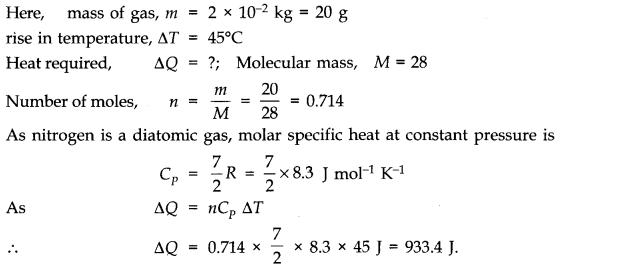
Question 12. 3 Explain why
(a) Two bodies at different temperatures T1 and T2, if brought in thermal contact do not necessarily settle to the mean temperature (T1 + T2)/2 ?
(b) The coolant in a chemical or nuclear plant (i.e., the liquid used to prevent different parts of a plant from getting too hot) should have high specific heat. Comment.
(c) Air pressure in a car tyre increases during driving. Why?
(d) The climate of a harbour town is more temperate (i.e., without extremes of heat and cold) than that of a town in a desert at the same latitude. Why?
Answer: (a) In thermal contact, heat flows from the body at higher temperature to the body at lower temperature till temperatures become equal. The final temperature can be the mean temperature (T1+ T2)/2 only when thermal capacities of the two bodies are equal.
(b) This is because heat absorbed by a substance is directly proportional to the specific heat of the substance.
(c) When car is driven, some work is being done on types in order to overcome dissipative forces of friction and air resistance etc. This work done is transformed into heat, due to which temperature of the car types increases.
(d) The climate of a harbour town is more temperate (neither too hot nor too cool) due to formation of sea breeze at day time and land breeze at night time as already explained in Chapter 11.
Question 12. 4 A cylinder with a movable piston contains 3 moles of hydrogen at standard temperature and pressure. The walls of the cylinder are made of a heat insulator, and the piston is insulated by having a pile of sand on it. By what factor does the pressure of the gas increase if the gas is compressed to half its original volume?
Answer: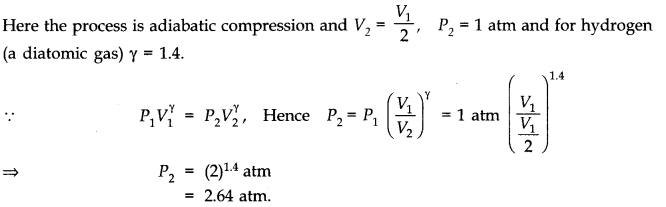
Question 12. 5 In changing the state of a gas adiabatically from an equilibrium state A to another equilibrium state B, an amount of work equal to 22.3 J is done on the system. If the gas is taken from state A to B via a process in which the net heat absorbed by the system is 9.35 cal, how much is the net work done by the system in the latter case? (Take 1 cal = 4.19 J)
Answer: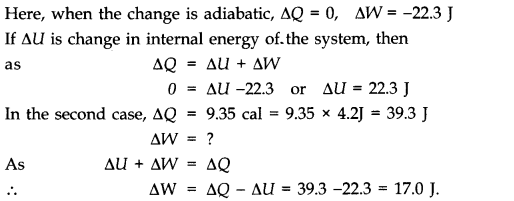
Question 12. 6 Two cylinders A and B of equal capacity are connected to each other via a stopcock. A contains a gas at standard temperature and pressure. B is completely evacuated. The entire system is thermally insulated. The stopcock is suddenly opened. Answer the following:
(a) What is the final pressure of the gas in A and B ?
(b) What is the change in internal energy of the gas?
(c) What is the change in the temperature of the gas?
(d) Do the intermediate states of the system (before settling to the final equilibrium state) lie of its P-V-T Surface?
Answer: (a) Since the final temperature and initial temperature remain the same,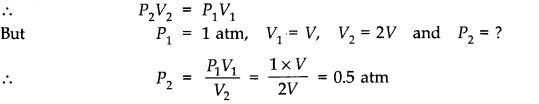
(b) Since the temperature of the system remains unchanged, change in internal energy is zero.
(c) The system being thermally insulated, there is no change in temperature (because of free expansion)
(d) The expansion is a free expansion. Therefore, the intermediate states are non equilibrium states and the gas equation is not satisfied in these states. As a result, the gas can not return to an equilibrium state which lie on the P-V-T surface.
Question 12. 7 A steam engine delivers 5.4 x 108 J of work per minute and services 3.6 x 109 J of heat per minute from its boiler. What is the efficiency of the engine? How much heat is wasted per minute?
Answer: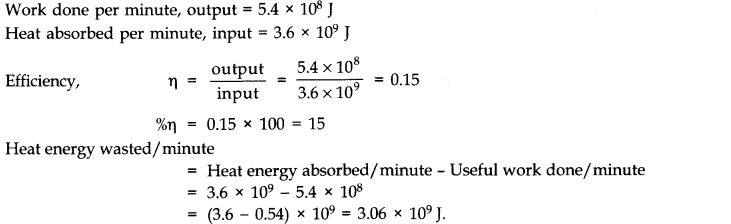
Question 12. 8 An electric heater supplies heat to a system at a rate of 100 W. If system performs work at a rate of 75 Joules per second. At what rate is the internal energy increasing?
Answer: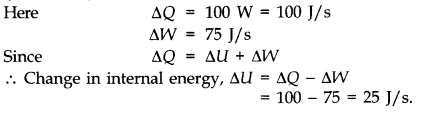
Question 12. 9 A thermodynamic system is taken from an original state to an intermediate state by the linear process shown in Fig.Its volume is then reduced to the original value from E to F by an isobaric process. Calculate the total work done by the gas from D to E to F.
Answer: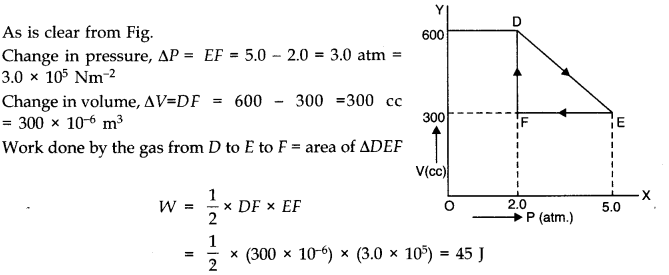
Question 12. 10 A refrigerator is to maintain eatables kept inside at 9 °C, if room temperature is 36 °C. Calculate the coefficient of performance.
Answer: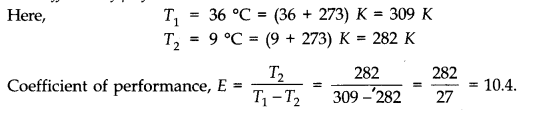
We hope the NCERT Solutions for Class 11 Physics Chapter 12 Thermodynamics help you. If you have any query regarding NCERT Solutions for Class 11 Physics Chapter 12 Thermodynamics, drop a comment below and we will get back to you at the earliest.








0 Comments
Please do not use any abuse language or Enter any spam Link in the comment box.